How Does Surface Tension Break . energy is required to break these intermolecular bonds. 9 years ago. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. Unravel the enigma of surface tension and adhesion. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Since water has a high surface tension, much energy is required to separate its. Water has a high surface tension because the water molecules. Equivalently, it can be stated as surface energy in ergs. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.
from www.sciencefacts.net
energy is required to break these intermolecular bonds. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water has a high surface tension because the water molecules. Since water has a high surface tension, much energy is required to separate its. Equivalently, it can be stated as surface energy in ergs. Unravel the enigma of surface tension and adhesion. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. 9 years ago.
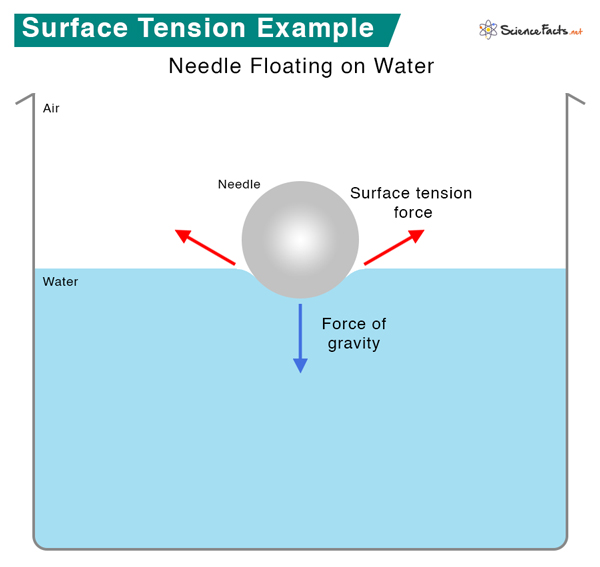
Surface Tension Definition, Examples, and Unit
How Does Surface Tension Break surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic. energy is required to break these intermolecular bonds. Water has a high surface tension because the water molecules. Unravel the enigma of surface tension and adhesion. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Equivalently, it can be stated as surface energy in ergs. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Since water has a high surface tension, much energy is required to separate its. 9 years ago.
From bio.libretexts.org
4.5.1.3 CohesionTension Theory Biology LibreTexts How Does Surface Tension Break energy is required to break these intermolecular bonds. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. surface tension,. How Does Surface Tension Break.
From www.youtube.com
Breaking Surface Tension with an Infiltration Surfactant Precision How Does Surface Tension Break surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic. energy is required to break these intermolecular bonds. Water has a high surface tension because the water molecules. 9 years ago. Equivalently, it can be stated as surface energy in ergs. Since water has a high surface tension, much. How Does Surface Tension Break.
From www.slideserve.com
PPT Marine Biology Lesson 3 PowerPoint Presentation, free download How Does Surface Tension Break Since water has a high surface tension, much energy is required to separate its. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic. Water has a high surface tension because the water molecules. surface tension is the energy, or work, required to increase the surface area of a liquid. How Does Surface Tension Break.
From www.reddit.com
Surface Tension r/Swimming How Does Surface Tension Break Water has a high surface tension because the water molecules. Equivalently, it can be stated as surface energy in ergs. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic.. How Does Surface Tension Break.
From www.diplomageeks.com
Define capilirity, viscosity, coefficient of viscosity and surface How Does Surface Tension Break 9 years ago. energy is required to break these intermolecular bonds. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water has a high surface tension because the water molecules. surface tension in water might be good at performing tricks, such as being able to. How Does Surface Tension Break.
From www.whatdowedoallday.com
Surface Tension Experiment Water Drop Races How Does Surface Tension Break surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic. 9 years ago. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since water has a high surface tension, much energy is required to separate its. Water has. How Does Surface Tension Break.
From indepest.com
Surface Tension Indepest How Does Surface Tension Break Water has a high surface tension because the water molecules. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Since water has a high surface tension, much energy is required to separate its. energy is required to break these intermolecular bonds. surface tension, property of a. How Does Surface Tension Break.
From sciencing.com
How Does Detergent Break Surface Tension? Sciencing How Does Surface Tension Break Since water has a high surface tension, much energy is required to separate its. Water has a high surface tension because the water molecules. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. 9 years ago. Unravel the enigma of surface. How Does Surface Tension Break.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts How Does Surface Tension Break Water has a high surface tension because the water molecules. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic. surface tension in water might be good at performing. How Does Surface Tension Break.
From www.sexiezpix.com
Ppt Motivation For Studying Fluid Mechanics Powerpoint SexiezPix Porn How Does Surface Tension Break 9 years ago. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Since water has a high surface tension, much energy is required to separate its. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic. Water has. How Does Surface Tension Break.
From people.csail.mit.edu
Codimensional Surface Tension Flow on Simplicial Complexes How Does Surface Tension Break Equivalently, it can be stated as surface energy in ergs. Unravel the enigma of surface tension and adhesion. Since water has a high surface tension, much energy is required to separate its. energy is required to break these intermolecular bonds. 9 years ago. surface tension, property of a liquid surface displayed by its acting as if it. How Does Surface Tension Break.
From thumbpress.com
Breaking The Water Surface Tension How Does Surface Tension Break Unravel the enigma of surface tension and adhesion. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. 9 years ago. Equivalently, it can be stated as surface energy. How Does Surface Tension Break.
From cartoondealer.com
Surface Tension Explanation Vector Illustration Diagram CartoonDealer How Does Surface Tension Break surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. Equivalently, it can be stated as surface energy in ergs. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Unravel the. How Does Surface Tension Break.
From thesciencegeeks.com
Break the Barrier A Surface Tension Quiz The Science Geeks How Does Surface Tension Break Since water has a high surface tension, much energy is required to separate its. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic. Water has a high surface tension. How Does Surface Tension Break.
From www.youtube.com
Surface tension On / OFF YouTube How Does Surface Tension Break surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Unravel the enigma of surface tension and adhesion. energy is required to break these intermolecular bonds. Equivalently, it can be stated as surface energy in ergs. 9 years ago. surface tension in water might be good. How Does Surface Tension Break.
From www.pinterest.com
Perfect shot of a swimmer just before the water’s surface tension is How Does Surface Tension Break Water has a high surface tension because the water molecules. energy is required to break these intermolecular bonds. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension in water might be good at performing tricks, such as being able to float a paper clip. How Does Surface Tension Break.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) How Does Surface Tension Break energy is required to break these intermolecular bonds. Unravel the enigma of surface tension and adhesion. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. surface tension, property of a liquid surface displayed by its acting as if it were. How Does Surface Tension Break.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit How Does Surface Tension Break Equivalently, it can be stated as surface energy in ergs. 9 years ago. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water has a high surface tension. How Does Surface Tension Break.